Hepatitis B: Information for Health Professionals - NIP8954
Information for health professionals on managing babies born to hepatitis B (HBsAg) positive parents.
The full resource:
Hepatitis B
Information for health professionals on the management of babies born to HBsAg positive pregnant people
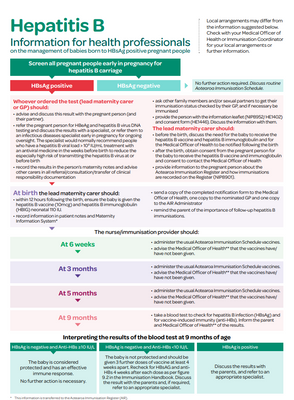
Screen all pregnant people early in pregnancy for hepatitis B carriage
HBsAg negative
- No further action required
- Discuss routine Aotearoa Immunisation Schedule
HBsAg positive
Whoever ordered the test (lead maternity carer or GP) should:
- advise and discuss this result with the pregnant person (and their partner)
- refer the pregnant person for HBeAg and hepatitis B virus DNA testing and discuss the results with a specialist, or refer them to an infectious diseases specialist early in pregnancy for ongoing oversight. The specialist would normally recommend people who have a hepatitis B viral load > 108 IU/mL treatment with an antiviral medicine in the weeks before birth to reduce the especially high risk of transmitting the hepatitis B virus at or before birth
- record the results in the person’s maternity notes and advise other carers in all referral/consultation/transfer of clinical responsibility documentation
- ask other family members and/or sexual partners to get their immunisation status checked by their GP, and if necessary be immunised
- provide the person with the information leaflet (NIP8952/HE1402) and consent form (HE1446). Discuss the information with them.
The lead maternity carer should:
- before the birth, discuss the need for the baby to receive the hepatitis B vaccine and hepatitis B immunoglobulin and for the Medical Officer of Health to be notified following the birth
- after the birth, obtain consent from the pregnant person for the baby to receive the hepatitis B vaccine and immunoglobulin and consent to contact the Medical Officer of Health
- provide information to the pregnant person about the Aotearoa Immunisation Register and how immunisations are recorded on the Register (Aotearoa Immunisation Register information flyer - NIP8901 – HealthEd).
At birth the lead maternity carer should:
- within 12 hours following the birth, ensure the baby is given the hepatitis B vaccine (10mcg) and hepatitis B immunoglobulin (HBIG) neonatal 110 IU
- record information in patient notes and Maternity Information System1,
- send a copy of the completed notification form to the Medical Officer of Health, one copy to the nominated GP and one copy to the AIR Administrator
- remind the parent of the importance of follow-up hepatitis B immunisations.
At 6 weeks of age, the nurse/immunisation provider should:
- administer the usual Aotearoa Immunisation Schedule vaccines.
- advise the Medical Officer of Health2 that the vaccines have/have not been given.
At 3 months of age, the nurse/immunisation provider should:
- administer the usual Aotearoa Immunisation Schedule vaccines.
- advise the Medical Officer of Health2 that the vaccines have/have not been given.
At 5 months of age, the nurse/immunisation provider should:
- administer the usual Aotearoa Immunisation Schedule vaccines.
- advise the Medical Officer of Health2 that the vaccines have/have not been given.
At 9 months of age, the nurse/immunisation provider should:
- take a blood test to check for hepatitis B infection (HBsAg) and for vaccine-induced immunity (anti-HBs). Inform the parent and Medical Officer of Health2 of the results.
Interpreting the results of the blood test at 9 months of age
HBsAg is negative and Anti-HBs ≥10 IU/L
The baby is considered protected and has an effective immune response.
No further action is necessary.
HBsAg is negative and Anti-HBs < 10 IU/L
The baby is not protected and should be given 3 further doses of vaccine at least 4 weeks apart. Recheck for HBsAg and anti-HBs 4 weeks after each dose as per Figure 9.2 in the Immunisation Handbook. Discuss the result with the parents and, if required, refer to an appropriate specialist.
HBsAg is positive
Discuss the results with the parents, and refer to an appropriate specialist.
1 This information is transferred to the Aotearoa Immunisation Register (AIR). If the process is electronic it is sent automatically. If the process is manual, LMC should ensure the completed enrolment is sent to the local district AIR administrator.
2 The Medical Officer of Health will identify whether information should be provided to the local Immunisation Coordinator. For further information see the Hepatitis B chapter of the Immunisation Handbook 2020. If you need further assistance please contact your local Medical Officer of Health or Immunisation Coordinator.
Hepatitis B vaccine and immunoglobulin
Storage, administration and dose
Hepatitis B immunoglobulin
Hepatitis B immunoglobulin (HBIG) neonatal 110 IU.
See the HBIG package insert for further information.
Storage
-
- Protect from light.
- This solution is freeze sensitive (do not freeze.)
- Storage above or below the recommended +2°C to +8°C will reduce potency.
- The solution must be stored in a fridge which is monitored daily to ensure the correct temperature of +2°C to +8°C is maintained.
Failure to do so may render the solution ineffective.
Administration and dose
-
- Hepatitis B immunoglobulin (HBIG) neonatal 110 IU.
- Allow the preparation to reach room temperature before administering to the infant.
- Use 25 G / 16 mm needle (or use the syringe and needle provided with the product).
- HBIG should be given slowly by the intramuscular route. Do not administer intravenously because of the potential for anaphylactic reactions.
- The lateral thigh is recommended for infants and young children.
- The Hepatitis B immunoglobulin may be given at birth at the same time as the Hepatitis B vaccine but should be given in the opposite lateral thigh.
Hepatitis B vaccine
Hepatitis B vaccine (Engerix®-B) 10 mcg in 0.5 mL
See the vaccine package insert for further information.
Storage
-
- This vaccine is freeze sensitive (do not freeze).
- Storage above or below the recommended +2°C to +8°C will reduce potency. In particular hepatitis B vaccine is very sensitive to colder temperatures.
- The vaccine must be stored in a fridge which is monitored daily to ensure the correct temperature of +2°C to +8°C is maintained.
- Failure to do so may render the vaccine ineffective.
Administration and dose
-
- Hepatitis B vaccine (Engerix®-B) 10 mcg.
- Shake well before withdrawal of vaccine from the vial.
- Do not mix with other vaccines.
- Change needle after drawing up prior to administration.
- Use 23-25 G / 16 mm needle.
- Give by intramuscular injection. Do not inject intravenously or intradermally.
- The lateral thigh is the recommended site for infants and young children. It should not be given in the buttock as this will result in lower seroconversion rates. Subcutaneous administration will result in increased local reaction. The Hepatitis B vaccine may be given at birth at the same time as the Hepatitis B immunoglobulin but should be given in the opposite lateral thigh.
Age & Vaccine
Birth:If you have any concerns, or need information about the vaccine or HBIG, please contact your local Medical Officer of Health or Immunisation Coordinator.
For more information, visit Health Information and Services